Korea Medical Device Labeling Requirements . Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in.
from www.slideshare.net
Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of.
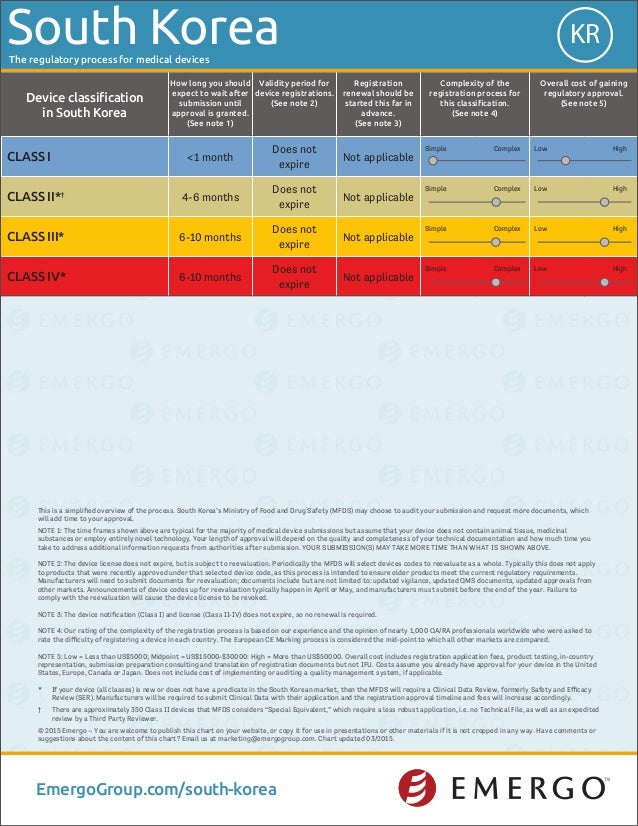
South Korea medical device approval chart Emergo
Korea Medical Device Labeling Requirements Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of.
From www.youtube.com
South Korea Medical Device Registration Chapter 1 Overview YouTube Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device. Korea Medical Device Labeling Requirements.
From www.youtube.com
South Korea Medical Device Registration Chapter 5 Registration YouTube Korea Medical Device Labeling Requirements Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance. Korea Medical Device Labeling Requirements.
From lifesciences.csoftintl.com
The EU MDR Labeling Journey Best Practices for Navigating the Latest Korea Medical Device Labeling Requirements Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import. Korea Medical Device Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Korea Medical Device Labeling Requirements Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance. Korea Medical Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device. Korea Medical Device Labeling Requirements.
From www.vigiservefoundation.org
Device Vigilance Requirements in South Korea INOPP Forum VigiServe Korea Medical Device Labeling Requirements Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web new packaging. Korea Medical Device Labeling Requirements.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of. Korea Medical Device Labeling Requirements.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Korea Medical Device Labeling Requirements Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device. Korea Medical Device Labeling Requirements.
From www.kmdia.or.kr
KOREA MEDICAL DEVICES INDUSTRY ASSOCIATION Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web new packaging. Korea Medical Device Labeling Requirements.
From www.youtube.com
South Korea Medical Device Registration Chapter 6 Final Steps YouTube Korea Medical Device Labeling Requirements Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on. Korea Medical Device Labeling Requirements.
From www.presentationeze.com
FDA Medical Device Labeling requirements. PresentationEZE Korea Medical Device Labeling Requirements (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import. Korea Medical Device Labeling Requirements.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device. Korea Medical Device Labeling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Korea Medical Device Labeling Requirements (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import. Korea Medical Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the. Korea Medical Device Labeling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Korea Medical Device Labeling Requirements Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on. Korea Medical Device Labeling Requirements.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device production (krw 4 trillion and 350 billion) •in 2021, 53.9% recorded for in vitro diagnostic medical device import out of total. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the. Korea Medical Device Labeling Requirements.
From tsquality.ch
Deciphering South Korea Regulation for Medical Devices TSQuality.ch Korea Medical Device Labeling Requirements Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web medical device labeling requirements in korea are outlined by the minister of food and drug safety (mfds) in. (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical device. Korea Medical Device Labeling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Korea Medical Device Labeling Requirements (application) according to the pharmaceutical affairs act (hereinafter referred to as the act) and the act on the control of. Web medical devices exported to south korea are subject to new packaging and labeling requirements under guidance issued. Web new packaging and labeling requirements on medical devices imported to south korea that went into effect in january. Web medical device. Korea Medical Device Labeling Requirements.